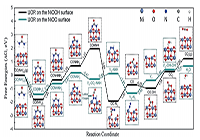
案例:The Gibbs free energy (DG) profiles calculated at the standard conditions and the simplified surface structures of the various reaction species along the reaction pathways of UOR on the NiOO and NiOOH surfaces.
说明:. As seen in Figure, the lattice oxygen atoms in the NiOOH surface do not involve in the reactions. While on the NiOO surface, the lattice oxygen atoms react with the *CONNH2 intermediate to form line-type adsorbed CO2 molecules, which may readily desorb from the surface of catalyst. As a result, on the NiOOH surface, the last step of CO2 desorption exhibits a potential-independent energy barrier of 0.90 eV. It is noted that the adsorption energy of a urea molecule on two adjacent Ni3+ ions on the NiOOH surface is 1.79 eV, which is similar to the binding energy of two CO2 molecules on two Ni3+ ions (0.90 2 = 1.80 eV). This strongly implies that the CO2 desorption and the subsequent re-generation of Ni3+ active sites may be a slow process in the catalytic cycle.
来源文献:https://doi.org/10.1002/anie.201909832
第一性原理计算的基本思想是将多个原子构成的体系看成是由多个电子和原子核组成的系统,并根据量子力学的基本原理对问题进行最大限度的“非经验性”处理。它只需要5个基本常数(m0,e,h,c,kB)就可以计算出体系的能量和电子结构等物理性质。它可以确定已知材料的结构和基础性质,并实现原子级别的精准控制,是现阶段解决实验理论问题和预测新材料结构性能的有力工具。并且,第一性原理计算不需要开展真实的实验,极大地节省了实验成本,现已被广泛应用于化学、物理、生命科学和材料学等领域。
适合的研究方向包括但不限于:催化、电池、半导体、金属材料、非金属材料、合金、纳米材料等
可以计算的体系包括但不限于:晶体、非晶、二维材料、表面、界面、固体等
常用软件:VASP,MS,CP2K,QE等
可以计算的内容包括但不限于:
材料的几何结构参数(如键长、键角、二面角、晶格常数、原子位置等)
材料的电子结构信息(如电荷密度、电荷差分密度、态密度、能带、费米能级、功函数、ELF等)
材料的光学性质(如介电常数等)
材料的力学性质(如弹性模量等)
材料的磁学性质(如磁导率等)
材料的晶格动力学性质(如声子谱等)
材料的表面性质(如吸附能,催化计算等)
复合材料的性质(异质结等内容)等等