案例效果展示:
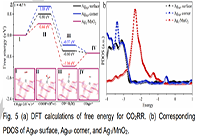
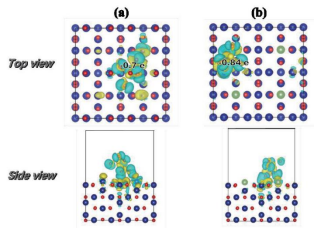
To further investigate the origin of catalytic activity of Ag1/MnO2 catalyst, we calculate the free energy diagram of hydrogen evolution reaction (HER) and CO2RR pathways for both AgNP (corner and surface) and Ag1/MnO2. The Gibbs free energies and related structures of key reaction intermediates, including *COOH, *CO, and *H in the pathways of CO2RR and HER are showing in Fig. 5a, and S17-S20, respectively. Compared to Ag atoms on the surface or corner of AgNP, Ag1/MnO2 exhibited lower formation energy of *COOH at U = - 0.7 V estimated to 0.44 eV. By comparison, the formation energies at the corner and surface of AgNP were calculated as 1.08 eV and 0.8 eV, respectively. This indicated a good CO2RR performance of Ag1/MnO2 than AgNP/MnO2 with Ag atoms as the only active sites. The DFT results of the first step for CO2 activation was consistent with CO2 temperature programmed desorption (CO2-TPD) results in Fig. S21. The reason for this was related to the overpotential, which was primarily determined by the formation energy of *COOH. It should be pointed out that the final step of *CO intermediate desorption was associated with the energy rising step over Ag1/MnO2 since *CO strongly bind to single Ag atoms, which agreed with the CO2-TPD results in Fig. S21b.[24] Besides, the free energy diagrams of HER in Ag1/MnO2 and AgNP indicated that HER could occur only over Ag1/MnO2 at very elevated overpotentials. This led to the remarkable performance of Ag1/MnO2 with FECO reaching up to 90% from -0.7 to -0.9 V.
来源文献:DOI: 10.1002/anie.202014718
第一性原理计算的基本思想是将多个原子构成的体系看成是由多个电子和原子核组成的系统,并根据量子力学的基本原理对问题进行最大限度的“非经验性”处理。它只需要5个基本常数(m0,e,h,c,kB)就可以计算出体系的能量和电子结构等物理性质。它可以确定已知材料的结构和基础性质,并实现原子级别的精准控制,是现阶段解决实验理论问题和预测新材料结构性能的有力工具。并且,第一性原理计算不需要开展真实的实验,极大地节省了实验成本,现已被广泛应用于化学、物理、生命科学和材料学等领域。
适合的研究方向包括但不限于:催化、电池、半导体、金属材料、非金属材料、合金、纳米材料等
可以计算的体系包括但不限于:晶体、非晶、二维材料、表面、界面、固体等
常用软件:VASP,MS,CP2K,QE等
可以计算的内容包括但不限于:
材料的几何结构参数(如键长、键角、二面角、晶格常数、原子位置等)
材料的电子结构信息(如电荷密度、电荷差分密度、态密度、能带、费米能级、功函数、ELF等)
材料的光学性质(如介电常数等)
材料的力学性质(如弹性模量等)
材料的磁学性质(如磁导率等)
材料的晶格动力学性质(如声子谱等)
材料的表面性质(如吸附能,催化计算等)
复合材料的性质(异质结等内容)等等