(1)定义及意义
第一性原理中的电荷密度指的是电子密度。根据哥本哈根解释,量子在空间某处出现的概率密度等于波函数的平方。由此可以得到体系中电子在任一点处的密度。
(2)结果案例展示
电子密度总是倾向于在原子核附近聚集,通过观察电子密度无法得到任何有价值的信息。因此关心的几乎总是差分密度。
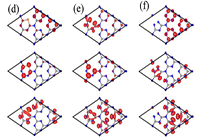
案例:partial charge density
说明:To study the variation of the active sites in the S-doped and vacancy-defected g-C3N4, we calculated partial charge density corresponded to VBM, gap states and CBM. Fig. 3(d) shows the partial charge density of the SN2-doped system. For the CBM, the electrons mainly come from the C and N2 atoms in two of the intact triazine units, while the VBM is mainly contributed by the N2 and S atoms in the S-doped triazine unit, aided by the N2 atoms around the doped site. Additionally, the gap states originate from the C atoms and S atom in the S-doped unit. The partial charge density of VN2-defected g-C3N4 is plotted in Fig. 3(e). One can see that the CBM and gap states are both contributed by the defected triazine unit, while the VBM is contributed by all N2 atoms in the whole calculational supercell. Fig. 3(f) shows the case of g-C3N4 with C-vacancy. Clearly, the partial charge density corresponded to the CBM and VBM are similar to that in the pristine case, which are mainly contributed by the C (N2) and N2 atoms in the three of intact triazine units, respectively, while the gap states originate from the triazine unit with C-vacancy. Overall, the partitioned electron distribution corresponded to the CBM and VBM in the three systems, especially SN2-doped g-C3N4, should be beneficial to suppressing the recombination of photogenerated electron-hole pairs.
来源文献:https://doi.org/10.1016/j.apsusc.2018.12.085
第一性原理计算的基本思想是将多个原子构成的体系看成是由多个电子和原子核组成的系统,并根据量子力学的基本原理对问题进行最大限度的“非经验性”处理。它只需要5个基本常数(m0,e,h,c,kB)就可以计算出体系的能量和电子结构等物理性质。它可以确定已知材料的结构和基础性质,并实现原子级别的精准控制,是现阶段解决实验理论问题和预测新材料结构性能的有力工具。并且,第一性原理计算不需要开展真实的实验,极大地节省了实验成本,现已被广泛应用于化学、物理、生命科学和材料学等领域。
适合的研究方向包括但不限于:催化、电池、半导体、金属材料、非金属材料、合金、纳米材料等
可以计算的体系包括但不限于:晶体、非晶、二维材料、表面、界面、固体等
常用软件:VASP,MS,CP2K,QE等
可以计算的内容包括但不限于:
材料的几何结构参数(如键长、键角、二面角、晶格常数、原子位置等)
材料的电子结构信息(如电荷密度、电荷差分密度、态密度、能带、费米能级、功函数、ELF等)
材料的光学性质(如介电常数等)
材料的力学性质(如弹性模量等)
材料的磁学性质(如磁导率等)
材料的晶格动力学性质(如声子谱等)
材料的表面性质(如吸附能,催化计算等)
复合材料的性质(异质结等内容)等等